Korea Eundan Multivitamin Product Recalled Due to Excess Iodine Detection
It has been reported that a popular multivitamin product has been recalled due to the detection of iodine levels exceeding safety standards.
On the 17th, the Ministry of Food and Drug Safety announced that Korea Eundan Healthcare Co., Ltd. manufactured and sold the "Multivitamin All-in-One" product, which has been deemed non-compliant and is currently subject to sales suspension and recall measures.
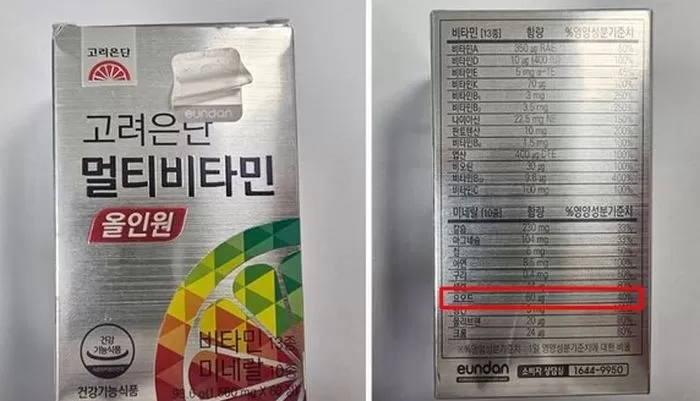
Excess iodine content was detected in the product.
While the product's iodine content is indicated as 60 μg, the actual detected amount is 129.6 μg, which corresponds to 216% of the standard specifications.
The standard for iodine is set at 80-150% of the labeled amount.
The recalled product has an expiration date of February 10, 2027, and is packaged in a form of 60 tablets, each weighing 1560 mg. The barcode number is confirmed to be 8809497531729.

The Ministry of Food and Drug Safety has requested sellers holding the product to immediately suspend sales and advised consumers to return the product to the place of purchase.
Meanwhile, iodine is an essential nutrient for growth and development in our bodies, playing a critical role in overall metabolism, particularly in regulating the secretion of thyroid hormones that balance energy in the body.
However, excessive intake can lead to thyroid disorders such as hyperthyroidism and thyroiditis, so caution is advised.
Image source: Ministry of Food and Drug Safety, 자료 사진 for understanding the article / gettyimagesbank


